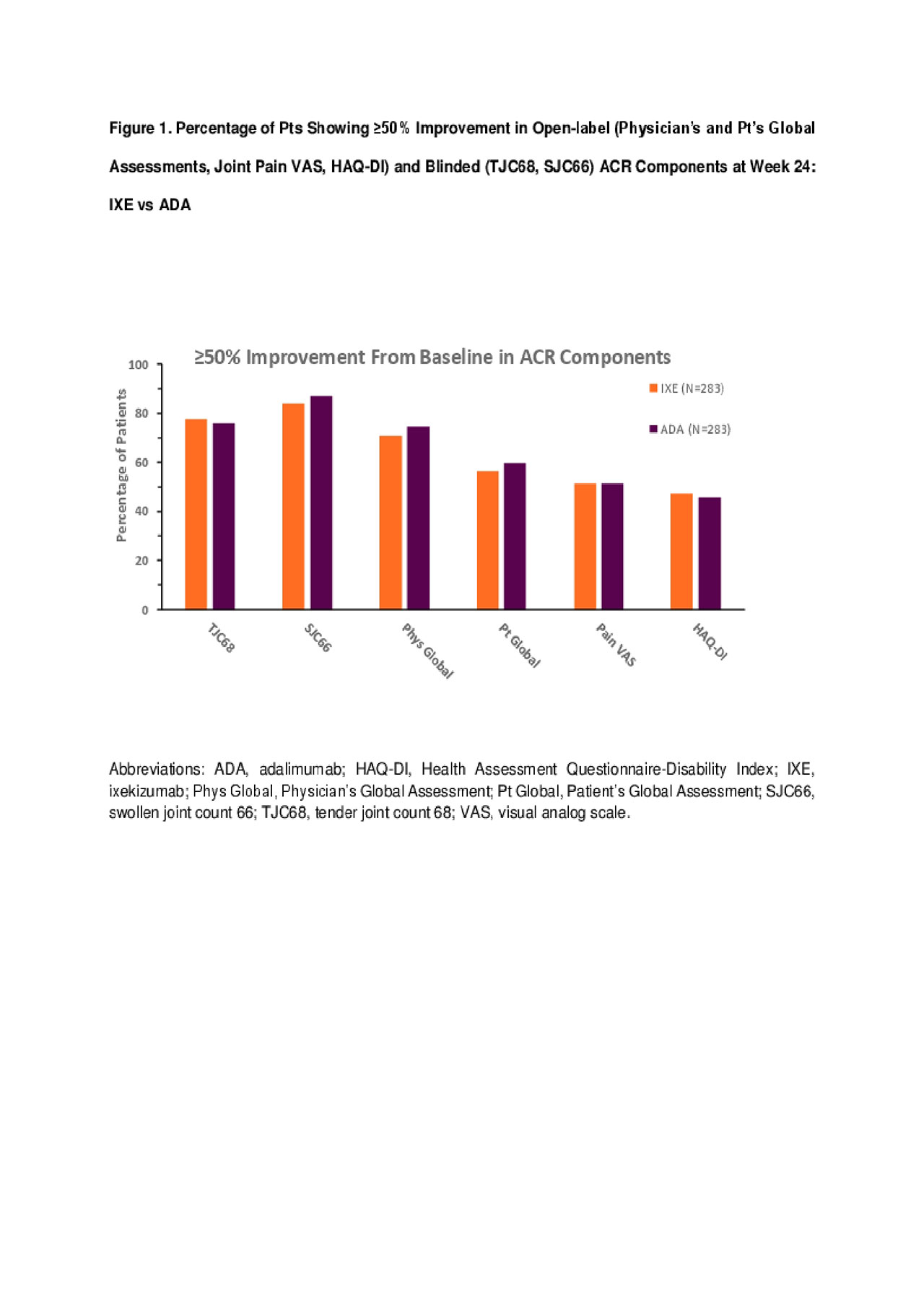
Figure 1. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology

Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial - The Lancet

Figure 3. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology

Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)
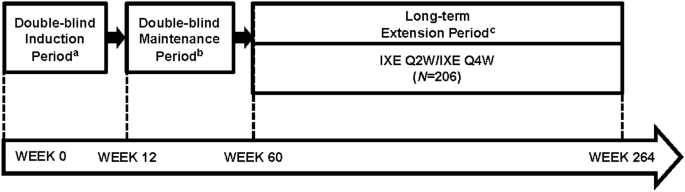
Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials | SpringerLink

Study design for administration of ixekizumab via a prefilled syringe... | Download Scientific Diagram

A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis - ScienceDirect

Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52 | Annals

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - Journal of the American Academy of Dermatology

These highlights do not include all the information needed to use TALTZ safely and effectively. See full prescribing information for TALTZ.TALTZ ( ixekizumab) injection, for subcutaneous useInitial U.S. Approval: 2016

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect

Lilly's Taltz® (ixekizumab) is the First IL-17A Antagonist to Receive U.S. FDA Approval for the Treatment of Non-Radiographic Axial Spondyloarthritis (nr-axSpA)

Long‐term efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis: subgroup analyses of an open‐label, phase 3 study (UNCOVER‐J) - Okubo - 2019 - Journal of the